We use cookies on our website to improve the website and your experience.
Read moreVeltis® Technology
Innovation is at the heart of everything we do. Our proprietary drug delivery technology platform, Veltis®, uses engineered recombinant albumin to enhance albumin’s intrinsic characteristics for optimal therapeutic performance of your API.
Veltis® - Enhancing the body’s natural drug delivery system
Biopharmaceuticals offer several advantages over conventional pharmaceuticals. However, many promising biopharmaceuticals, such as protein and peptides drug candidates fail to produce the desired clinical effect because of rapid inactivation, removal from the body and/or inadequate delivery at site of action. These shortcomings of biologics often result in the requirement for both high and more frequent dosing, with the consequent risk of side effects and reduced treatment compliance.
Our Veltis® technology platform is developed to address these key challenges by leveraging albumin – the human body’s own delivery system. Considered the natural carrier for distribution of pharmaceuticals around the human body, albumin is inherently designed to engage with a range of chemical and biological compounds.
Through subtle modifications to the albumin molecule, we have further enhanced its already remarkable intrinsic characteristics, while preserving its unique biological mechanisms and stable nature. In effect, our Veltis technology platform can transform genetically fused and/or chemically conjugated drugs into more effective, safe and convenient therapeutics.
These therapeutic benefits are achieved through the multiple functional attributes of the pioneering Veltis technology platform:
- Half-life Extension: Through an unprecedented high affinity to the neonatal Fc receptor (FcRn), Veltis enables significantly prolonged duration of action of associated compounds, opening the possibilities for enhanced therapeutic effect, while reducing dose frequency.
- Bi-Specificity: Coupled with multiple conjugation sites and the possibility for both N- and C-terminal fusion, the Veltis technology platform allows for the creation of bispecific compounds and the possibility to engage multiple targets at once.
- Site-specific delivery: Driven by passive targeting, a preferential accumulation of Veltis associated compounds can be achieved at diseased tissue, such as in inflamed tissue and solid tumors. Through site specific delivery it is possible to maximize dosing without compromising safety.
- Increased payload capacity: Beyond the naturally available thiol-conjugation site on albumin, the Veltis variants offers multiple conjugation sites, providing the opportunity to modulate the drug-to-albumin ratio (DAR) for optimal impact.
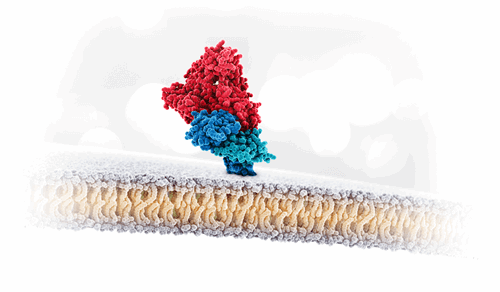
Albumin interaction with FcRn receptor
A validated & patent protected technology platform
With more than 30 years of experience with recombinant human albumin and pioneering albumin based technologies, we have created the world’s most extensive patent estate concerning engineered albumin variants; its receptor engagement, manufacturing and related technologies.
Altogether this has enabled the creation of a partnered pipeline of programs at various stages, ranging from discovery to market, and across multiple therapeutic areas. (Pipeline).
Overcoming short drug half-life using albumin
Various factors contribute to poor patient compliance; a challenge that is particularly observed for pharmaceuticals which are rapidly cleared from the body, ultimately requiring a higher or more frequent dosing.
By linking biopharmaceuticals to albumin, their short serum half-life can be significantly extended. As an example, the therapeutic efficacy of peptides is often limited due to their fast removal from the body - in some cases within minutes. Yet, through the association with albumin, their effect may be extended to weeks.
The long serum half-life of albumin is achieved in part by its size, 66 kDa, which prevents clearance through the kidney, and by its interaction with the neonatal Fc receptor (FcRn). Together, this results in albumin’s natural long half-life of approx. three weeks.


Albumin Recycling: Albumin is naturally recycled via an FcRn-driven endosomal sorting pathway. Albumin binds to FcRn under acidic pH and all non-receptor bound proteins goes through lysosomal degradation, where after albumin is released from the cell again.
Extending drug half-life with the Veltis® technology
Our scientists have interrogated the interaction of albumin with FcRn and found that by selective modification of a few amino acids, the half-life of albumin can be dramatically extended, creating the foundation of the Veltis technology platform. Demonstrated in in vivo studies, our engineered albumin variants have shown more than double the half-life of native sequence albumin, along with its ability to enhance the therapeutic effect of associated drugs.
Achieved benefits with Veltis technology:
- Broad design flexibility - Covalent linkage of candidates to Veltis can be achieved by genetic fusion, either in yeast or mammalian cells, or chemical conjugation.
- Improved dosing regimen – Through prolonged exposure, Veltis enables the possibility to reduce dose frequency and/or concentration of pharmaceuticals otherwise requiring un-economical and sub-optimal dosing.
- Simpler formulation – By extending the time a pharmaceutical is held within the therapeutic window, drug developers can move from inconvenient administration routes and formulations to more patient friendly solutions.
Veltis enabled half-life extension already benefitting patients
Utilizing our native sequence albumin fusion technology, two products have already been approved in major markets. As an example, our Veltis technology is utilized by CSL Behring in their Idelvion® product, a recombinant coagulation factor IX-albumin fusion protein, revolutionizing treatment for hemophilia B patients through increased protection.
Tissue specific delivery by harnessing the targeting ability of albumin
Systemic delivery of drugs gives rise to several challenges, such as uniform biodistribution and inadequate specificity to diseased tissue. Traditionally, this has been compensated by administrating a large total dose to achieve high local concentration, which unfortunately may result in non-specific toxicity and other adverse effects.
The effectiveness of active pharmaceutical compounds greatly increases when these are delivered directly to their site of action. To attain site specific delivery, various drug targeting platforms have been developed over the years. Albumin stands out due to its intrinsic ability to accumulate in specific disease tissue.
Inflamed tissue and tumor accumulation using recombinant albumin
Tumor accumulation by albumin:
Albumin’s propensity for tumor accumulation has been well demonstrated. Exposing growing tumors to color- or radioactive labelled albumin demonstrates how albumin specifically targets various tumor tissue. Read more on this here
Several reasons for this specific tumor targeting of albumin have been reported:
- It has been observed that serum albumin levels deplete in cancer patients, suggesting that albumin is taken up as a food source by the tumors. Read more here
- The ability of albumin to accumulate in tumors is furthermore attributed to passive targeting in areas with leaky vasculature, the so-called Enhanced Permeability Effect. Learn more here
- Engagement of albumin with cellular receptors, such as SPARC, Gp60 and Gp30, have been hypothesized to enhance tumor uptake through a receptor mediated mechanism. Learn more here
Although the exact reasons for albumin’s ability to accumulate in tumors have not been clarified, a number of albumin–cytotoxin constructs have been shown to be suitable for tumor delivery, with the most prominent being Celgene’s Abraxane®, approved by the FDA for use against advanced breast, pancreatic and non-small cell lung cancer.
Inflamed tissue accumulation by albumin:
Following similar mechanisms for the targeting ability of albumin against tumors, site specific targeting by albumin against inflamed tissue has been demonstrated using a collagen induced arthritis model. Read more here
The prospect of using albumin as a carrier for targeting active pharmaceutical compounds to diseased tissue has been achieved by the fusion of an interleukine-1 receptor antagonist to albumin. Read more here
Enhanced drug targeting at diseased tissue using Veltis® technology platform
When using albumin as a drug targeting platform, our recombinant human albumin Recombumin® and its drug delivery technology Veltis® holds many advantages over plasma derived human serum albumin (HSA), both in terms of its performance, versatility, safety and controllability. When employing Albumedix’ technologies it is therefore possible to target tumors and/or inflamed tissues by albumin site-specific delivery of one or more drugs for increased potency or bispecificity.
Bispecific delivery using recombinant human albumin as a versatile platform
For many new biotherapeutics, the ability to bind two different receptors or ligands and elicit the required biological responses simultaneously, is critical for full therapeutic effect. It is therefore vital that the employed delivery platform offers effective control for the generation of such bispecific entities.
Multiple molecular formats have been developed using antibodies, with varying success in creating a stable and homogeneous bispecific molecule that can be easily scaled for commercial manufacturing.
Albumin offers an attractive alternative to bispecific antibodies with its stable and simple molecular architecture. Coupled with the added half-life and payload capacity benefits of our engineered albumin variants (Veltis®), this alternative protein scaffold design offers a versatile platform for bispecific drug delivery:
- Optimized drug performance, compliance and safety profile through modulatation of drug half-life using our engineered albumins displaying a significantly higher affinity for the natural receptor of albumin, FcRn.
- Generation of bispecific albumin drug conjugates with a DAR (Drug Albumin Ratio) of up to three, achievable through site-specific conjugation to albumin variants with strategically placed conjugation sites.
- Tissue specific delivery at sites of inflammation and/or solid tumors through passive and active targeting.
- The intrinsic structural nature of albumin makes it a simple and stable molecular format for development of homogeneous bispecifics, with an established and scalable manufacturing path.
- Broad design flexibility for creation of bispecifics, through fusion and conjugation to engineered albumin variants, while preserving the high stability of albumin.
Broad design flexibility for creation of albumin-based bispecifics
Engineered recombinant human albumins from Albumedix have several options available for the design of bispecific molecules. By combining site specific conjugation to one or more thiols, with N and/or C-terminal fusion to albumin, it is possible to design a multitude of bispecific albumin drug constructs.

Bi-specific albumin drug construct design by fusion and conjugation to Veltis® albumin variants
Bispecific delivery design:
- Albumin fusion: We have, with success, made both C and N-terminal albumin fusions individually and combinations thereof.
- Conjugation at Cys34: Albumin conjugation enables the generation of other drugs, over natural amino acids in single linear design, as required for fusion proteins. Learn more here
- Conjugation at engineered surface thiol sites: Our engineered albumin, as to make it carry two additional free cysteine thiols, enabling the generation of albumin drug conjugates with a DAR (Drug Albumin Ratio) of up to three, read more here.
It should be noted that the positions of these cysteines are distributed as to minimally influence the albumin’s ability to bind to the FcRn, whereby not compromising the achievable half-life extension of improved albumin binders, even when multiple drugs are loaded on the albumin protein. Learn more about half life extension here
