We use cookies on our website to improve the website and your experience.
Read morePartnered Pipeline
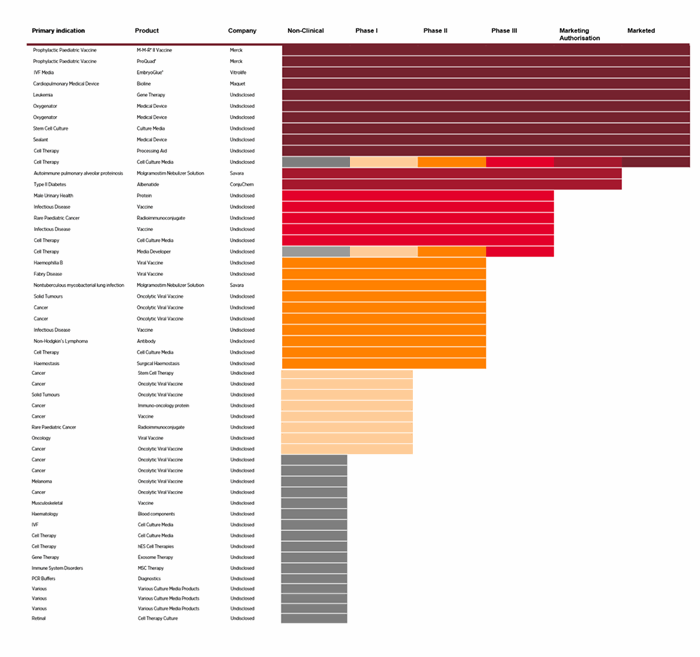
Albumedix’ albumin-based technologies are both clinically and commercially validated in a number of albumin-enabled pharmaceutical programs by partners. These programs include all from preclinical candidates to marketed products spanning a wide range of therapeutic indications. Together these programs and partnerships demonstrate the value and applicability of Albumedix’ recombinant human albumin products and technologies.
Recombumin® is used in the manufacture of Merck & Co’s M-M-R® II and ProQuad® childhood vaccines
The vaccines are used in immunization against measles, mumps, rubella, and varicella virus infections in children 12 months through 12 years of age.
Since Recombumin was introduced in the M-M-R® II vaccine in 2006 more than >200 million doses have been administered in children worldwide.

Veltis® is used in CSL Behrings IDELVION®
Hemophilia B, is a genetic disorder caused by missing or defective factor IX. IDELVION® is recombinant factor IX replacement therapy.
Veltis® technology delivers the extended half-life to factor IX enabling a reduced frequency of injections; Idelvion is the only Hemophilia B Therapy with up to 14-day dosing intervals. Patients are able to maintain factor IX activity levels over a longer period of time, allowing greater freedom from frequent infusions.

You are always welcome to contact us to learn more about Albumedix' products and technologies