We use cookies on our website to improve the website and your experience.
Read moreCOVID-19 Vaccines, Diagnostics & Medical Devices
Pure, consistent & safe; the key to unlocking fast access to market for your life-saving vaccines, medical devices or diagnostics. Armed with the highest quality albumin commercially available and 35 years' of expertise in how to use it, Albumedix can work with you to overcome your COVID-19 development challenges, enabling you to reach patients faster.
Get in touchCOVID-19: Knowledge Hub
The COVID-19 pandemic has affected the entire world, not least of which the pharmaceutical industry.
In Albumedix, we are proud to support multiple customers active in the fight against the Coronavirus, developing and delivering medical devices, diagnostics or vaccines which will help patients worldwide.
Recombumin® for COVID-19 Vaccine Stabilization
The natural properties of albumin can help to overcome critical challenges in the ongoing COVID-19 vaccine development due to its ability to protect from sheer stress, prevent surface adsorption and provide thermal stability to sensitive vaccines. Albumedix rHA has been used in both marketed vaccines and a range of clinical and pre-clinical programs already.
In all pharmaceutical development speed is always of paramount importance, yet never has this been truer in quickly getting safe and efficient COVID-19 vaccines to patients worldwide. The key to speed in vaccine development is multi-faceted and relies on many factors. Albumedix' albumin products can be a key ingredient to facilitate speed within several points in production and development.
- Avoid regulatory hurdles – Albumedix Recombumin® products comes with extensive regulatory documentation and inhouse regulatory experts supporting our customers with their filings and interaction with the regulatory agencies, ensuring a faster and smoother path through clinic and to market.
- Maintain stability during manufacturing, storage and delivery – Vaccines are like other biopharmaceuticals vulnerable to environmental stress both physical and chemical strain as well as temperature changes. Through its natural properties, Albumedix stabilizes vaccines and minimize vaccine inactivation by preventing aggregation, surface adsorption and keeping thermal stability.
- Reduce excipient evaluation time – Albumin is a very versatile stabilizer which can be used across the manufacturing process as well as for a variety of viral structures supporting the demand for higher volume manufacturing of vaccines. Recombumin® have been shown to be applicable across a broad range of vaccine constructs, such as live attenuated vaccines, whole-inactivated, sub-unit vaccines, viral vector vaccines, virus-like particles (VLP’s) and enveloped vaccines.
The choice of excipient can consequently key to obtaining safe and fast access to market. Recombumin® is a multi-functional and validated excipient and can ensure consistency and maintain the stability and efficiency of COVID-19 vaccines which are so important in order to ensure patients receive optimal treatment with maximal effect as quickly as possible.
Albumedix recombinant human albumin products has already been commercially proven with its use in more than 70 million doses worldwide in Merck’s childhood vaccines; MMR®II and ProQuad®. Recombumin® is a proven and safe excipient for stabilization of vaccine products.
Learn more about albumin in vaccines and virus-based therapies on our dedicated vaccine application page, in our recent scientific blog or in our Recombumin® for Vaccine Stabilization infographic. For scientific journal articles discussing the use of rHA in vaccine development, access our Scientific library.
Recombumin® for the Improvement of Diagnostics
Due to the unsurpassed purity of Albumedix´ recombinant human albumin products (Recombumin® product portfolio), we are able to support our diagnostics customers and enable more effective COVID-19 tests, including PCR, RT-PCR, LAMP and RT-LAMP diagnostic test kits.
Benefits of Recombumin® include:
- Save preparation time – Run samples with lower levels of template DNA or from “dirtier” samples.
- Reduce loss of reagents – Prevent non-specific adsorption of reagents to glass capillaries or PCR tubes.
- Avoid false-negative results – Bind inhibitors due to high level of free binding sites.
- No concern of unexpected pathogens – Confirmed virus free products
Read more details on the benefits of Recombumin® in diagnostics in our recent Scientific blog or download the Recombumin® for Improving Diagnostics infographic.
Why choose recombinant and not BSA or HSA?
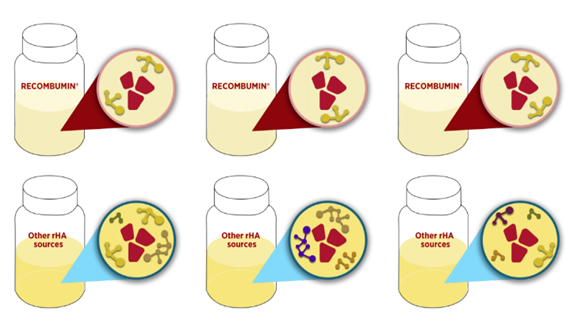
Amongst the functional benefits that albumin provide to diagnostic tests, purity of the product is key. Due to the controlled cGMP manufacturing of Albumedix´ recombinant human albumins and 25+ years of manufacturing experience, Recombumin® is the purest albumin product on the market.
Both Bovine Serum Albumin (BSA) and Human Serum Albumin (HSA) are obtained from blood and with varying sources. This means that the product will contain a number of unidentified impurities in the formulation and sticking to the albumin molecules. With less free binding sites on the albumin the product will not be as efficient in binding the PCR inhibitors. These impurities will furthermore greatly vary from batch to batch, making consistency in the final diagnostic more difficult.


Challenges in COVID-19 diagnostics
Essential considerations in the development and production of diagnostics is reducing cost while maximizing productivity. One area however that is becoming increasingly important, especially regarding COVID-19 testing is reliability, faster testing, and speed of results.
The benefits that Recombumin offers diagnostic developers addresses exactly these needs. The purity and consistency of Recombumin® allows for shorter preparation times, lower occurrence of false-negatives and a safer product.
Recombumin® for the Improvement of Medical Devices
In light of the COVID-19 pandemic, the need for safe, biocompatible, lifesaving medical devices such as ECMO machines has increased exponentially. A key consideration in the manufacture and regulation of these medical devices is a suitable coating, which should be biocompatible and biostable whilst offering excellent lubricity, thermal stability, and protection. The ideal coating should positively impact performance, efficacy and safety of the medial device whilst ensuring no adverse response is elicit in the patient.
Albumin is the natural solution, being the most abundant protein in human blood it is not a foreign substance to humans and as such does not tricker any immune response. When used as a medical device coating it therefore creates a biological interface which is fully biocompatible. Because Albumedix albumin products are recombinantly produced, and not sourced directly from plasma, they offer the naturally occurring biocompatibility and benefits of albumin, but from a consistent, pure and safe source. Albumedix recombinant human albumin products enhance the safety further, being entirely human- and animal- free they reduce the risk of unexpected pathogens to zero and the extremely high batch-to-batch consistency in manufacturing provides reliable peace of mind.
To learn more about how Albumedix are supporting in the development of Medical Devices in connection to the COVID-19 pandemic, you can read this statement issued in collaboration with Chalice Medical.
Alternatively, you can visit our dedicated Medical Devices application page, read our recent scientific blog article or download our Recombumin® for Medical Devices Infographic.
Albumedix Technical Insights to COVID-19
Albumedix Chief Technology Officer, Phil Morton, discusses the technical challenges to be considered when developing a COVID-19 vaccine. Phil draws on his own experience in the biopharmaceutical industry spanning more than 25 years, as well as insights based on years of technical customer support and application data development. This episode covers everything from the characterization of the SARS-CoV2 virus, to the differences between the various types of vaccines, to specific manufacturing challenges in vaccine development. Phil addresses live-attenuated vaccines, inactivated vaccines, subunit vaccines, virus-like particles and gene-based vaccines. Finally, Phil dives into how the stability issues of these vaccines may be addressed using recombinant human albumin.
Regulatory perspectives on the development of COVID-19 vaccines
Harriet Edwards, Director of Regulatory Affairs in Albumedix, is responsible for regulatory filings of Albumedix' products as well as the extensive regulatory customer support we provide alongside our product sales. In the following episode, Harriet discusses the traditional clinical stages of vaccine development and the regulatory requirements throughout the process. The development process of the COVID-19 vaccines have been far from traditional in their timelines, so Harriet also delves into how this has been handled from a regulatory perspective and the challenges the industry and regulators have faced. Finally, Harriet adds her perspective on how this year has affected the future of regulations.
Commercial outlook
As Vice President of Commercial Operations, Joanna Hay spends every day in Albumedix helping customers with their questions, understanding the challenges they are facing and the industry in which they operate. This year has been no exception and with new types of vaccines reaching patients and the speed in which they are being developed, manufactured, and distributed challenges and urgent needs to find solutions are especially critical. Joanna reviews the landscape of COVID-19 vaccines in development and reflects on what this tells us about market trends and the future of vaccine developments. Finally, Joanna reviews the challenges faced by these vaccines especially focusing on the more novel gene-based vaccines.
Panel Discussion
To wrap up our COVID-19 focused AX-TV series, we asked our 3 in-house experts how they think this pandemic will affect the future of vaccine development. The Albumedix Director of Regulatory Affairs, Chief Technology Officer and European Business Development Director share their thoughts, and further to this, consider the most exciting treatment trend or innovation in the vaccine development space in general.
Enquire about Recombumin® products
Please contact bd.nottingham@Sartorius.com for any enquiries.
